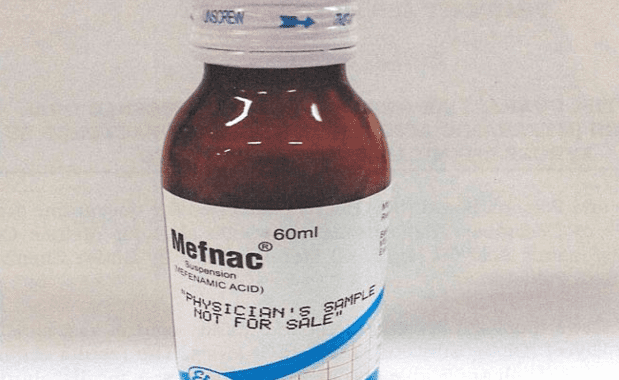
The Pharmacy and Poisons Board (PPB) has lifted a suspension it ordered on December 11, 2024, of a drug used for mild pain relief.
In a statement on Monday, March 17, 2025, the board revealed that following thorough assessments, the Mefnac Oral Suspension had met the required standards.
“The Pharmacy and Poisons Board (“the Board”) has lifted the quarantine order issued on 11th of December 2024 through a public alert for Mefnac Oral Suspension (Mefenamic Acid 50 Mg/5 MI) Manufactured by Efroze Chemical Industries Pvt Ltd, Pakistan,” the board said.
“The decision follows thorough investigations and quality control assessments for Diethylene Glycol (DEG)/Ethylene Glycol (EG) levels. The results confirm that the product meets all applicable specifications and is safe for distribution and use.”
“The Board urges the public to report any suspected cases of sub-standard medicines or adverse drug reactions to the nearest healthcare facility or the Pharmacy and Poisons Board,” the statement read.
On December 11, 2024, PPB quarantined the drug and banned its distribution, sale or use by members of the public over suspicion of contamination with Diethylene Glycol (DEG)/Ethylene Glycol (EG) at levels above the acceptable limits.

“The Pharmacy and Poisons Board (“the Board”) informs the public of a Quarantine Order issued for Mefnac Oral Suspension (Mefenamic Acid 50 Mg/5 MI) Manufactured by Efroze Chemical Industries P Pvt Ltd, Pakistan,” the board said.
Suspicion of contamination
“This action has been taken due to suspected contamination with Diethylene Glycol (DEG)/Ethylene Glycol (EG) at levels above the acceptable limits. In light of this, the Board advises all pharmaceutical outlets, healthcare and members of the public to immediately facilities, healthcare professionals, and members of the public to immediately quarantine the product and stop the further distribution, sale, issuance, or use until further communication from the Board.”
Mefnac oral suspension is normally used in the treatment of headaches, migraines, toothache, nerve pains ans menstrual cramps.
During the same period of December 11, 2024, the Pharmacy board also flagged an unregistered anti-cancer drug currently in circulation in the Kenyan market.
The board stated that they discovered that the Floracil 1000 (Fluorouracil 1000mg/2ml) Injection was in the market despite failing to undergo their approval.
“During routine post-market surveillance (PMS) activities, the Board identified an unregistered and substandard product, Floracil 1000 (Fluorouracil 1000mg/2ml) Injection, manufactured by Bruck Pharma PVT LTD, India,” the board said.
“In light of this, the Board strongly cautions the public and healthcare professionals against the trade, distribution, wholesale, retail, issuance, dispensing, use, or administration to patients of this product.“